If your UK Certificate of Free Sale (CFS) has been rejected by a foreign authority, the cause is very often not the Apostille itself.
In most real-world cases, the rejection is triggered by incorrect or low-standard UK solicitor certification wording — even when a UK Apostille has already been issued.
This guide explains exactly why UK CFS documents are rejected, how solicitor certification, UK Apostille, and embassy legalisation work together, and how to ensure your documents are accepted for medical device and regulated exports.
Why UK Certificate of Free Sale Is Commonly Rejected
Foreign regulators review UK CFS documents from a regulatory-risk perspective, not an administrative one.
They look for one key element:
Who is professionally responsible for confirming the authenticity of this document?
If that responsibility is unclear, the CFS is rejected — regardless of whether an Apostille is attached.
The Most Common Mistake: Incorrect Solicitor Certification Wording
Many rejected UK CFS files contain solicitor wording such as:
“I hereby certify that the attached documents are produced to me as printouts …”
This wording confirms only one thing:
that someone handed the solicitor a printed document.
It does not confirm:
- the document’s official source
- its authenticity
- its consistency with MHRA records
For medical devices, pharmaceuticals, cosmetics, and regulated products, this wording is insufficient and routinely rejected.

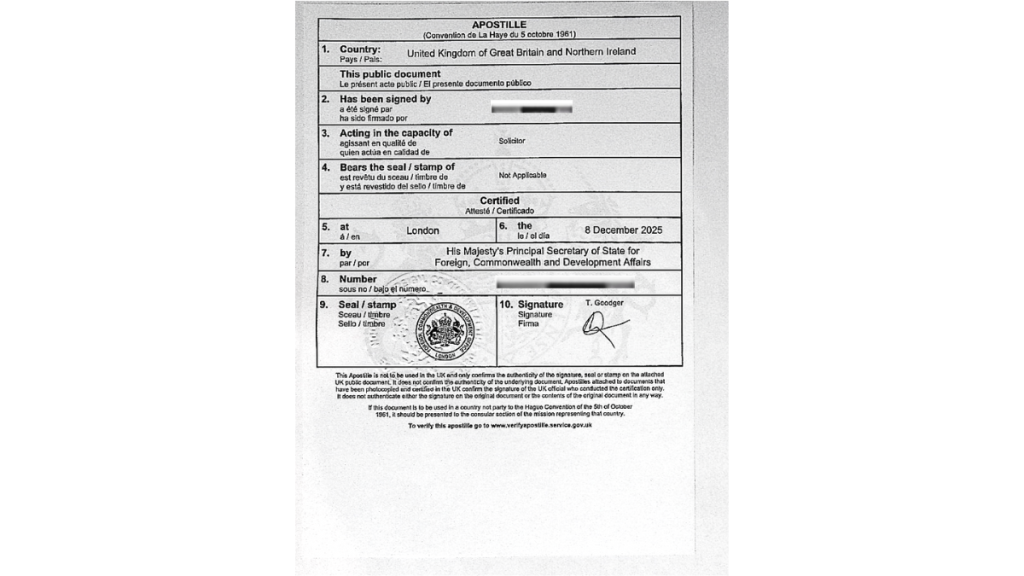
Why the UK Apostille Does Not Fix Poor Solicitor Certification
A UK Apostille issued by the Foreign, Commonwealth & Development Office (FCDO) does not verify document content.
It only verifies:
- the identity of the signer
- the authority of that signer
If the solicitor certification is weak, the Apostille merely authenticates a legally hollow statement.
This is why many exporters are surprised when a document with an Apostille is still rejected.
Why MHRA Certificate of Free Sale Cannot Be Apostilled Directly
A Certificate of Free Sale issued by the Medicines and Healthcare products Regulatory Agency (MHRA):
- is issued as an electronic PDF
- has no wet signature
- has no physical seal
Because the FCDO can only Apostille a verifiable signature, the MHRA CFS cannot be Apostilled directly.
A UK solicitor or notary must first convert the electronic CFS into a certifiable legal document.
What Proper UK Solicitor Certification Must Confirm
High-standard solicitor certification should clearly state that the solicitor has:
- reviewed the UK CFS
- verified its consistency with MHRA records
- confirmed the document’s authenticity
- accepted professional responsibility for that confirmation
This is the single most important step in the entire UK CFS legalisation process.
Low-cost or “fast” services often skip this responsibility — which is why rejections occur.

Correct UK Certificate of Free Sale Legalisation Process
Step 1 — MHRA Issues the CFS
Electronic PDF format.
Step 2 — UK Solicitor Certification
An SRA-regulated, FCDO-recognised solicitor certifies the CFS using regulator-acceptable wording.
Step 3 — UK Apostille (FCDO)
The FCDO verifies the solicitor’s signature and issues the Apostille.
Step 4 — Embassy Legalisation (If Required)
Required only for non-Hague Apostille Convention countries.
Skipping or weakening Step 2 is the most common cause of rejection.
Hague vs Non-Hague Countries: The Simple Rule
Hague Apostille Convention countries
(e.g. India, Pakistan, Argentina)
→ Solicitor certification + UK Apostille
Non-Hague countries
(e.g. United Arab Emirates, Vietnam, Thailand)
→ Solicitor certification + UK Apostille + Embassy legalisation
In all cases, solicitor certification is the foundation.

Why “Minimum Standard” Certification Is a High-Risk Choice
Minimum-standard certification may appear cheaper, but it creates serious regulatory risk:
- no clear accountability
- no authenticity verification
- Apostille adds no real protection
For medical device exports, a rejected CFS can delay registration, shipment, and market entry for months.
How Ginkgo Advisory Helps with UK CFS Rejections
Ginkgo Advisory specialises in UK Certificate of Free Sale certification and legalisation for overseas regulatory use, particularly for medical devices and regulated products.
We assist clients by:
- reviewing rejected or high-risk solicitor certifications
- arranging proper UK solicitor certification with regulator-accepted wording
- coordinating UK Apostille (FCDO) applications
- handling embassy legalisation for non-Hague countries
- advising on country-specific regulatory expectations before submission
Our focus is not speed alone, but regulatory acceptance.
Final Takeaway for Exporters
If your UK Certificate of Free Sale is rejected overseas, the real issue is rarely:
“Do we have an Apostille?”
The real issue is:
“What exactly did the solicitor certify, and did they accept responsibility for authenticity?”
For regulated exports, professional solicitor certification determines whether the document survives regulatory scrutiny.
Contact Us

Address
167-169 Great Portland Street, 5/F, London

