Why UK CFS documents are rejected in Vietnam — and how to ensure acceptanceWhy UK CFS documents are rejected in Vietnam — and how to get them accepted the first time
If your UK MHRA Certificate of Free Sale (CFS) is rejected in Vietnam, the cause is usually simple and preventable.
In practice, rejection rarely happens because of the Apostille.
Instead, it happens because the UK solicitor certification is weak, unclear, or incomplete.
This article explains the Vietnam-accepted process in plain language. More importantly, it shows you how to avoid rejection before you submit.
Why Vietnam Authorities Require a UK MHRA Certificate of Free Sale
Vietnamese regulators must confirm that a regulated product is legally sold in its country of origin.
Therefore, they often request a Certificate of Free Sale issued by a recognised regulator.
In the UK, that regulator is the Medicines and Healthcare products Regulatory Agency (MHRA).
As a result, UK MHRA CFS documents are commonly required in Vietnam for:
- medical devices
- in vitro diagnostics (IVDs)
- cosmetics
- other regulated healthcare products
Why UK MHRA CFS Documents Are Rejected in Vietnam
Vietnamese authorities do not review documents as a checklist.
Instead, they assess regulatory responsibility.
So the key question is not:
“Is there an Apostille?”
It is:
“Who takes professional responsibility for confirming this document is authentic?”
If that responsibility is unclear, rejection follows — even when an Apostille is attached.
The Main Cause of Rejection: Weak Solicitor Certification
Most rejected submissions contain wording such as:
“I certify that this document was produced to me as a printout.”
This wording creates problems because it proves almost nothing.
Specifically, it does not confirm:
- where the document came from
- whether it matches MHRA records
- whether it is authentic
- whether the solicitor accepts responsibility
For regulated products, Vietnamese authorities frequently reject certifications written this way.

Why a UK Apostille Does Not Fix the Problem
Many applicants ask:
“The document has a UK Apostille — why was it still rejected?”
The answer is straightforward.
A UK Apostille issued by the Foreign, Commonwealth & Development Office (FCDO) does not verify document content.
It only confirms:
- who signed the document
- that the signer is authorised
Therefore, if the solicitor’s statement is weak, the Apostille only confirms a weak statement.
An Apostille cannot repair poor certification.
Why an MHRA Certificate of Free Sale Cannot Be Apostilled Directly
This step often causes confusion.
An MHRA CFS:
- is issued as an electronic PDF
- has no wet signature
- has no physical seal
Because of this, the FCDO cannot Apostille the MHRA document itself.
Instead, a UK solicitor or notary must certify the MHRA CFS first.
Only then can the Apostille be issued.
What Vietnam-Accepted Solicitor Certification Must Clearly State
For Vietnam, the solicitor’s wording must be clear, direct, and accountable.
Specifically, it must confirm that the solicitor has:
- reviewed the MHRA Certificate of Free Sale
- checked it against MHRA records
- confirmed its authenticity
- accepted professional responsibility for that confirmation
This step forms the foundation of the entire legalisation process.
When this foundation is strong, later steps usually succeed.

Correct UK MHRA CFS Legalisation Process for Vietnam
To avoid rejection, follow this order exactly:
Step 1 — MHRA Issues the CFS
The MHRA issues the Certificate of Free Sale electronically.
Step 2 — UK Solicitor Certification
An SRA-regulated, FCDO-recognised UK solicitor certifies the CFS using regulator-accepted wording.
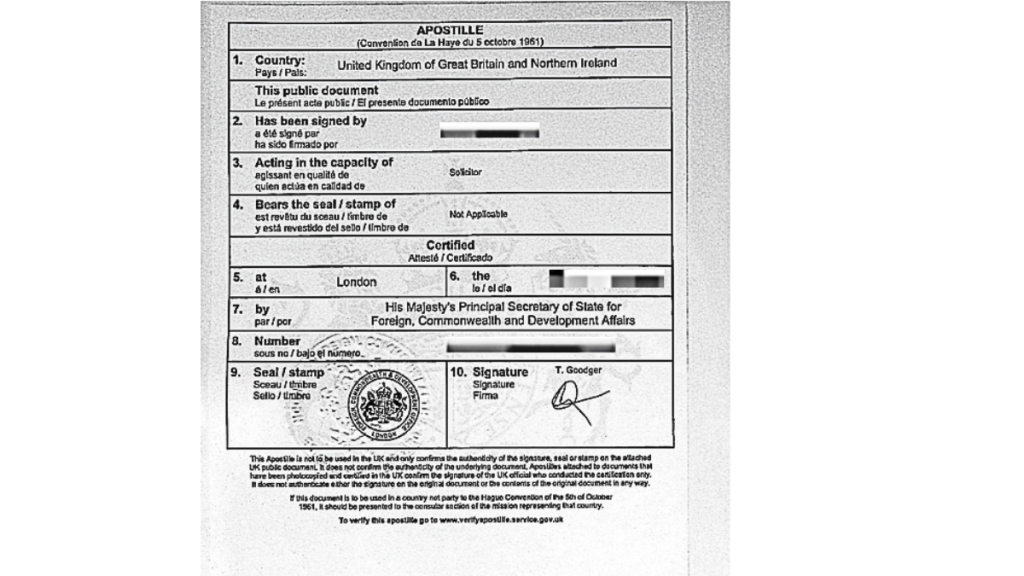
Step 3 — UK Apostille (FCDO)
The FCDO Apostille authenticates the solicitor’s signature.
Step 4 — Vietnam Embassy or Consular Legalisation
Because Vietnam is not a Hague Apostille Convention country, Vietnamese embassy or consular legalisation is required after the Apostille.
If Step 2 is weak, all later steps fail.

Why Low-Cost Certification Often Leads to Rejection
Minimal certification may look faster or cheaper. However, it increases regulatory risk.
Common issues include:
- no clear accountability
- no proof of authenticity checks
- lack of regulator confidence
As a result, rejection can delay:
- product registration
- customs clearance
- market entry
These delays often last months, not days.
Final Takeaway
When a UK MHRA Certificate of Free Sale is rejected in Vietnam, the issue is almost never:
“Did we get an Apostille?”
The real question is:
“Did the UK solicitor verify authenticity and accept responsibility clearly?”
If the answer is yes, acceptance usually follows.
If not, rejection is likely — regardless of how many stamps appear on the document.
Strong solicitor certification is the key.
Everything else depends on it.
How Ginkgo Advisory Helps
Ginkgo Advisory helps ensure UK MHRA Certificates of Free Sale are accepted in Vietnam and other non-Hague jurisdictions.
We focus on regulatory acceptance, not just document processing.
In practice, we:
- review MHRA CFS documents to identify Vietnam-specific risks
- arrange proper UK solicitor certification with accepted wording
- coordinate UK Apostilles through the FCDO
- manage Vietnamese embassy or consular legalisation
- correct weak or rejected certifications without restarting the process
As a result, your documents move through Vietnam regulatory review clearly, confidently, and without repeat submissions.
Contact Us

Address
167-169 Great Portland Street, 5/F, London

